Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you might wonder: bioequivalence, the scientific standard that proves a generic drug performs the same way in the body as its brand-name version. Also known as therapeutic equivalence, it’s not just a buzzword—it’s what keeps your prescriptions safe, effective, and affordable. If two drugs are bioequivalent, they release the same amount of active ingredient at the same speed. That means your body absorbs them the same way, whether you’re taking the name-brand version or the cheaper copy.
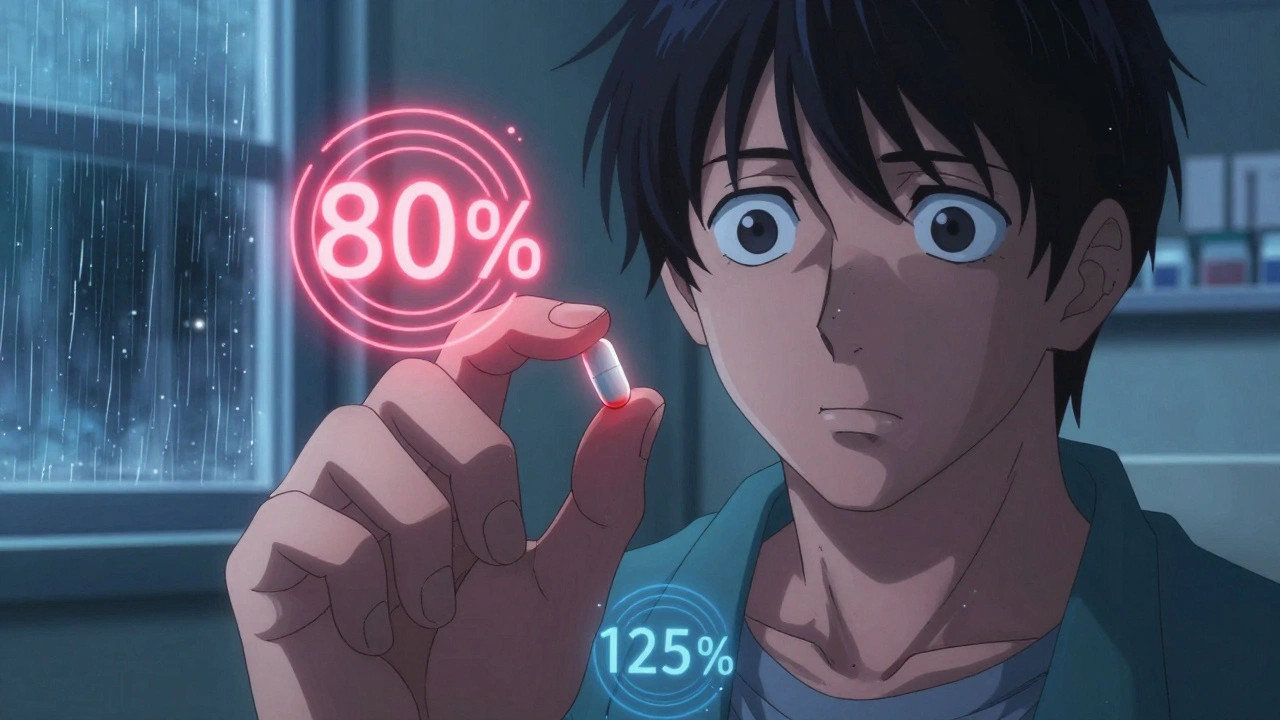
This isn’t guesswork. Regulators like the FDA and EMA require strict testing: blood levels of the drug are measured in volunteers after taking each version. If the results fall within a narrow range—usually 80% to 125% of the original drug’s levels—the generic is approved. No fluff, no shortcuts. That’s why you can trust a generic version of metformin, levothyroxine, or amlodipine to do the same job as the brand, even if the color or shape is different.
But bioequivalence isn’t just about chemistry. It’s about real-life outcomes. Take generic prescribing, the practice of doctors choosing lower-cost versions of drugs that meet bioequivalence standards. Also known as INN prescribing, it saves billions every year and helps patients stick to their treatment plans. When your doctor switches you from a brand to a generic, they’re relying on this science. And when you see a drug like Fildena or acyclovir priced much lower than the brand, bioequivalence is why that’s possible without sacrificing safety.
Some people worry about switching between generics or between brand and generic. But if both meet bioequivalence standards, the difference is negligible. That’s why studies show no increase in side effects or treatment failure when patients switch. The real risk? Not taking your meds because they’re too expensive. Bioequivalence closes that gap.
You’ll also see this concept tied to drug absorption, how quickly and completely a medication enters your bloodstream. Also known as pharmacokinetics, it’s the core of bioequivalence testing. Factors like food, stomach acid, or even the pill’s coating can affect absorption. That’s why generic makers must prove their version behaves the same under real conditions—not just in a lab.
Behind every generic drug you use, there’s a chain of science, regulation, and manufacturing precision. From India’s massive API production to the FDA’s review process, bioequivalence is the invisible guardrail that keeps your health on track. It’s why you can safely switch from one generic to another, why your insurance covers cheaper options, and why you’re not paying more for the same medicine just because of the brand name.
Below, you’ll find real-world guides that touch on this topic—whether it’s how to read drug labels, why generic prescribing works, or how to avoid double-dosing with cheaper meds. These aren’t theoretical articles. They’re practical tools built for people who need to understand what’s in their medicine—and why it works.