When you're told a generic drug is just as good as the brand-name version, you expect it to work the same way. But what happens when it doesn’t? For some patients, switching to a cheaper generic isn’t just inconvenient-it’s dangerous. Therapeutic failure isn’t rare. It’s happening in real time, with real people, and the consequences can be deadly.
Why a Generic Might Not Work
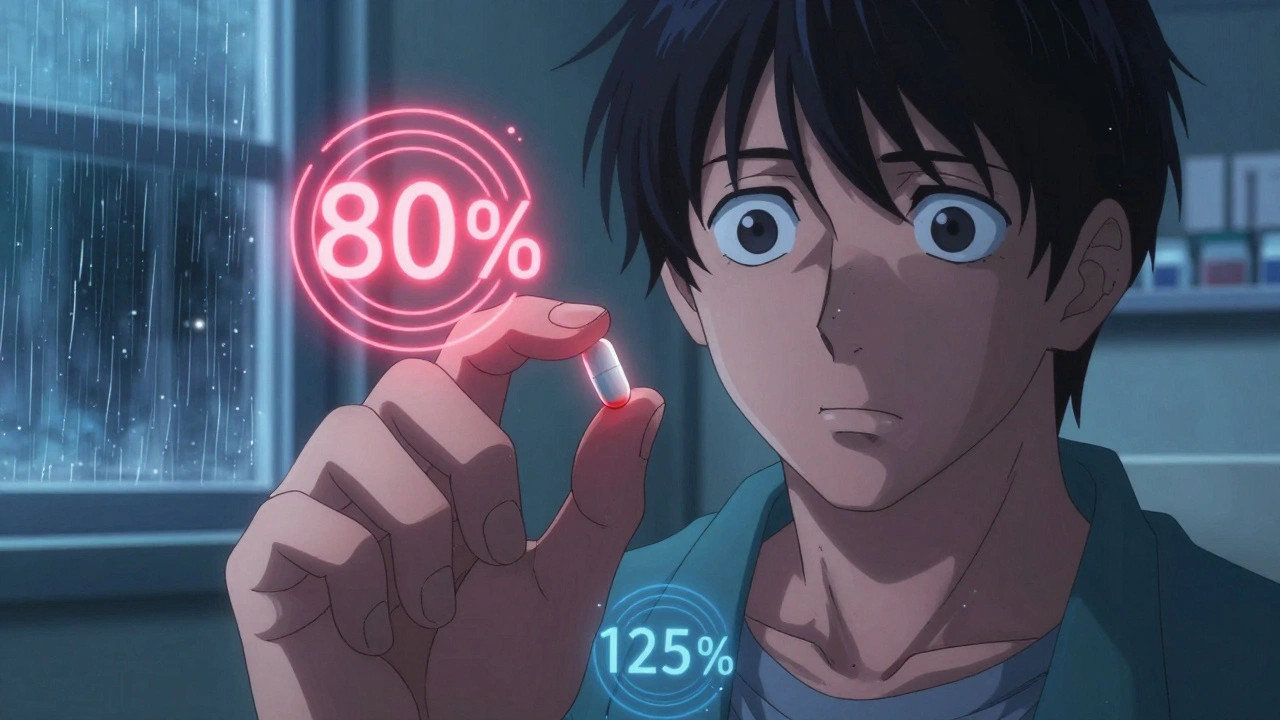
The FDA says generics must be bioequivalent to brand-name drugs. That means they’re supposed to deliver the same amount of active ingredient into your bloodstream at a similar rate. The acceptable range? Between 80% and 125% of the brand’s absorption. Sounds precise, right? But for drugs with a narrow therapeutic index-like warfarin, phenytoin, digoxin, or tacrolimus-that margin is too wide.For these drugs, even a 10% drop in absorption can mean the difference between treatment and toxicity. A patient on warfarin might go from safely preventing clots to bleeding internally. Another on tacrolimus after a transplant could reject their new organ. The FDA’s 80-125% rule was designed for common medications like antibiotics or blood pressure pills. It was never meant for drugs where the line between cure and catastrophe is razor-thin.
And it’s not just about the active ingredient. The fillers, coatings, and binders-called inactive ingredients-can change how a pill breaks down in your stomach. In 2013, the FDA pulled Budeprion XL, a generic version of Wellbutrin, after hundreds of patients reported severe side effects: anxiety, dizziness, even seizures. The problem? The generic used a different coating that released the drug too fast. The active ingredient was the same. The delivery system wasn’t.
The Hidden Problem: Manufacturing Inconsistencies
Most people assume all pills from the same batch are identical. They’re not. A 2025 investigation by STAT News found that in some generic chemotherapy drugs, pills from the same blister pack contained as little as 72% or as much as 112% of the labeled dose. That’s not a typo. That’s actual variation between pills taken minutes apart.Manufacturing flaws account for 31% of deficiencies in generic drug applications, according to Drug Patent Watch. Some common issues:
- Drug substance (the active ingredient) doesn’t dissolve properly
- Active ingredient clumps together instead of spreading evenly
- Chemical degradation from heat, moisture, or light-especially in poorly stored shipments
- Inadequate stability testing before release
One study found that only 4 out of 12 generic versions of a popular brand-name drug dissolved at the same rate. Some generics dissolved over three times faster. That’s not bioequivalence. That’s a lottery.

Real Cases, Real Harm
In 2024, a patient named Salberg, who had received a heart transplant, noticed her symptoms returning-fatigue, shortness of breath-after switching to a generic tacrolimus. Her doctor assumed her body was rejecting the organ. But when they switched her back to the brand name, her levels stabilized. The generic had released the drug too quickly, leaving her with dangerously low levels by the next dose.Multiple sclerosis patients in a 2024 study showed a clear pattern: those with stable disease were taking generics with 97-103% of the labeled dose. Those who relapsed? Their generics contained 91%, 82%, and even 73% of the required medication. No change in their condition. No change in their lifestyle. Just a switch in the pill they were given.
And it’s not just neurological or transplant drugs. In May 2024, Glenmark Pharmaceuticals recalled nearly 47 million doses of potassium chloride because the tablets weren’t dissolving. Patients with low potassium-often elderly or on diuretics-were at risk of fatal heart rhythms because the drug wasn’t getting into their bloodstream.
When the System Fails
The global generic drug market is worth $400 billion. Most of it comes from a handful of countries-India, China, and others-where regulatory oversight is weaker. The FDA inspects only a fraction of foreign factories. And when a problem is found, recalls are slow. Valsartan, losartan, irbesartan: all blood pressure meds recalled for cancer-causing nitrosamine contaminants. But how many others are still on shelves?Pharmacy Benefit Managers (PBMs)-the middlemen who negotiate drug prices for insurers-often push the cheapest generic, regardless of quality. They profit from volume, not outcomes. Doctors and pharmacists rarely have access to data on which generic manufacturer is reliable. So they prescribe based on cost, not performance.
One oncology pharmacist told STAT News: “We’ve seen patients stop responding to chemo. We switch drugs. We run tests. We never think it’s the generic-until it’s too late.”
What You Can Do
If you’re on a drug with a narrow therapeutic index-warfarin, lithium, levothyroxine, cyclosporine, phenytoin, or any transplant medication-here’s what to do:- Ask your doctor if your drug is an NTI drug. If yes, insist on staying on the brand name if your insurance allows.
- Don’t assume all generics are the same. If you’ve been switched and feel different-worse, not better-speak up. Track your symptoms.
- Ask your pharmacist: “Which manufacturer made this?” Write it down. If you’re switched again and feel off, ask if it’s the same maker.
- For high-risk drugs, request therapeutic drug monitoring. Blood tests can show if your levels are dropping.
- If you’re on chemotherapy, ask if your clinic uses generics-and which brand. Some oncology centers avoid generics entirely for this reason.
It’s not about being anti-generic. Most generics work fine. But for certain drugs, the stakes are too high to gamble.
What Needs to Change
Regulators need to tighten bioequivalence standards for NTI drugs. The FDA already uses a 90-111% range for some, but it’s not universal. It should be mandatory.Manufacturers must be held accountable. Batch-to-batch consistency should be publicly reported. Independent labs should test random generics on the market-like how food safety inspectors sample produce.
And patients need transparency. Pharmacies should list the manufacturer on the label. Not just the drug name. The company that made it. That way, if a batch fails, you know who to blame-and whether you’ve been given the same one before.
Until then, the burden falls on you. If your medication stops working, don’t assume your condition is worsening. Ask: Could it be the pill?”
Can a generic drug really be less effective than the brand name?
Yes, especially for drugs with a narrow therapeutic index-like warfarin, phenytoin, or tacrolimus. While generics must meet FDA bioequivalence standards (80-125% absorption), that range allows for significant variation in how the drug is absorbed. For some patients, even small differences can lead to treatment failure or toxicity. Cases have been documented where generic versions released medication too quickly or too slowly, causing serious side effects or loss of effectiveness.
Which generic drugs are most likely to fail?
Drugs with a narrow therapeutic index (NTI) are the highest risk. These include anticoagulants (warfarin), anti-seizure medications (phenytoin, carbamazepine), immunosuppressants (tacrolimus, cyclosporine), thyroid hormone (levothyroxine), and some heart medications (digoxin). Chemotherapy drugs, potassium chloride, and extended-release psychiatric medications like Concerta have also shown inconsistent performance in real-world use. The FDA has recalled specific generics in these categories due to improper dissolution or inconsistent dosing.
How do I know if my generic drug is causing problems?
Watch for unexplained changes in how you feel after switching to a generic: new side effects, worsening symptoms, or loss of control over your condition. For example, if you’re on warfarin and your INR levels suddenly fluctuate, or if your seizures return after switching to a generic anticonvulsant, it could be the medication. Keep a symptom log and talk to your doctor. Blood tests can confirm if drug levels are too low or too high.
Should I avoid all generic drugs?
No. Most generics work just as well as brand-name drugs. The risk is concentrated in a small subset of medications-mainly those with narrow therapeutic windows. For antibiotics, statins, or blood pressure pills, generics are usually safe and effective. The key is knowing which drugs are high-risk. If you’re unsure, ask your doctor or pharmacist. Don’t avoid generics out of fear-be informed about which ones matter most.
Can I request the brand-name drug instead of the generic?
Yes. You have the right to ask your doctor to write “Dispense as Written” or “Do Not Substitute” on your prescription. Insurance may require prior authorization or charge you a higher copay, but your health comes first. If you’ve had a bad reaction to a generic before, keep a record. Many doctors will support switching back if there’s a documented history of therapeutic failure.
Why aren’t more generics tested after they’re on the market?
Regulators test generics before approval, but post-market surveillance is limited. The FDA relies on patient and provider reports to catch problems. Independent testing is rare and expensive. Without mandatory batch testing and public reporting of results, unsafe generics can stay on shelves for years. Some experts call for random market testing, similar to how food or water safety is monitored-but no such system exists yet for most medications.
ruiqing Jane
December 1, 2025 AT 23:20This is terrifying. I’m on warfarin, and my INR spiked after my pharmacy switched me to a generic without telling me. I ended up in the ER. No one at the pharmacy even knew to warn me. I’ve since insisted on the brand-and yes, my insurance fought me. But I’d rather pay more than bleed out in my sleep.
Anthony Breakspear
December 2, 2025 AT 06:03Look, I get it-generic drugs save money. But this isn’t about saving a few bucks on a bottle of ibuprofen. This is about people getting poisoned by pills that look identical but act like wild cards. I’ve worked in hospice care for 12 years. I’ve seen patients crash after a generic switch-no change in diet, no new meds, just a different pill. And then? They’re told it’s ‘just progression.’ Bullshit. It’s the damn tablet.
And don’t even get me started on PBMs. They’re middlemen who don’t give a shit if you live or die, as long as they get their cut. The system’s rigged, and we’re the pawns.
Michael Campbell
December 4, 2025 AT 05:46China and India are poisoning us with fake medicine and the FDA is asleep at the wheel. This is why we need borders closed and American-made drugs only. No more foreign pills. Period.
Allan maniero
December 4, 2025 AT 23:05It’s funny how we assume all pills are created equal. I mean, we don’t expect two different brands of toilet paper to perform the same way-why do we assume two generics of tacrolimus will? The truth is, the body doesn’t care about the label-it cares about what’s actually in the pill and how it behaves in your gut. And right now, the system treats patients like lab rats in a cost-cutting experiment.
I’ve been on levothyroxine for 18 years. I’ve switched generics five times. Three of them made me feel like a zombie. The fourth? I lost 15 pounds in two weeks without trying. The fifth? Back to brand. I’m stable now. I don’t care if it costs $80 a month. I’d rather be alive and broke than dead and cheap.
william tao
December 5, 2025 AT 08:09Oh, here we go again. The ‘generic drug conspiracy.’ People need to stop blaming pills and start blaming themselves for not being more ‘informed.’ If you’re so worried, don’t take generics. But don’t scream ‘systemic failure’ because you didn’t read the fine print. You knew what you were signing up for.
Zoe Bray
December 6, 2025 AT 03:40Therapeutic failure in narrow therapeutic index (NTI) agents represents a significant pharmacokinetic and pharmacodynamic variance that is not adequately captured under current FDA bioequivalence paradigms. The 80–125% confidence interval for Cmax and AUC is statistically permissible for broad-spectrum agents but is clinically untenable for agents with steep dose-response curves. The absence of mandatory batch-to-batch pharmacopeial validation and real-time post-marketing bioavailability surveillance constitutes a critical gap in regulatory science.
Furthermore, the role of excipient heterogeneity in dissolution kinetics remains grossly undercharacterized in regulatory filings. The 2013 Budeprion XL recall was a sentinel event that was not systematically leveraged to reformulate NTI generic standards. This is not a market failure-it is a failure of regulatory epistemology.
Saravanan Sathyanandha
December 7, 2025 AT 03:02As someone from India, I’ve seen how these drugs are made. Factories run 24/7, workers don’t wear gloves, humidity isn’t controlled, and quality checks are a checkbox. I’m not saying all Indian generics are bad-but when a company can sell 50 million pills for $2, something’s wrong. We need global standards, not just US ones. Maybe if the FDA inspected factories like they do for food, things would change.
But also… patients need to speak up. If you feel different after a switch, tell your doctor. Write down the name of the manufacturer. Don’t just accept it. Your life is worth more than a copay.
Eddy Kimani
December 8, 2025 AT 08:00Wait-so you’re saying the FDA’s 80–125% range is too wide for drugs like warfarin? But didn’t they already adopt a tighter 90–111% range for some NTI drugs? Why isn’t that universal? Is it because manufacturers lobbied against it? Because if so, that’s not a science problem-it’s a corruption problem.
I’m a pharmacist. I’ve seen patients on phenytoin go from seizure-free to status epilepticus after a generic switch. We don’t test blood levels routinely unless someone crashes. That’s the problem. We’re waiting for disasters instead of preventing them.
alaa ismail
December 8, 2025 AT 11:28My dad was on digoxin. Switched to generic. Got dizzy, almost fell. We switched back. He’s fine now. No drama. Just a pill that didn’t work. Why is this so hard to fix?
Fern Marder
December 9, 2025 AT 15:43So… if I’m on lithium and my pharmacy switches me to a generic without telling me, and I start having tremors and confusion… it’s the pill? Not my stress? Not my diet? Not my thyroid? 😳
Victoria Graci
December 10, 2025 AT 10:27It’s not just about the drug-it’s about trust. We’re told medicine is science. But when the same pill, from the same pharmacy, can kill you or save you depending on which factory made it… what does that say about our faith in the system? We’ve turned healing into a commodity. And now we’re paying the price-not in money, but in lives.
Maybe the real question isn’t ‘Can generics fail?’ but ‘Why did we let this happen?’
Saurabh Tiwari
December 10, 2025 AT 12:57Paul Santos
December 12, 2025 AT 05:29Ah, the neoliberal pharmaceutical-industrial complex strikes again! 🤡 The commodification of human biology, where life-saving molecules are reduced to line items on a PBM spreadsheet. We’ve turned the sacred act of healing into a profit-driven algorithm. The FDA, beholden to corporate lobbyists, has abdicated its duty as guardian of the public trust. And yet-we are told to ‘be grateful’ for affordable medicine. What a grotesque irony.
Perhaps we must return to Hippocratic ideals… or at least demand that our pills be tested by independent labs, not by the same companies that profit from their failure. 🌍💊
Carolyn Woodard
December 13, 2025 AT 10:10There’s a deeper issue here: the lack of pharmacovigilance infrastructure in the U.S. We rely on passive reporting systems like MedWatch, which capture less than 1% of adverse events. Meanwhile, drug manufacturers are not required to conduct post-market bioequivalence audits. The result? A silent epidemic of therapeutic failure, masked as ‘patient noncompliance’ or ‘disease progression.’
Until we mandate real-time therapeutic drug monitoring for NTI drugs and require manufacturers to publish batch-level dissolution profiles, we are not practicing evidence-based medicine-we are practicing gambling.
Sandi Allen
December 14, 2025 AT 22:11THIS IS WHY WE NEED TO BAN FOREIGN DRUGS. THE FDA IS CORRUPT. THEY TAKE MONEY FROM CHINA. THEY LET THESE POISON PILLS BE SOLD. MY COUSIN DIED BECAUSE OF A GENERIC. I KNOW IT. I SAW THE BATCH NUMBER. IT WAS MADE IN INDIA. THEY’RE KILLING US. WE NEED TO SEIZE ALL FOREIGN DRUGS. NOW. 🚨💣