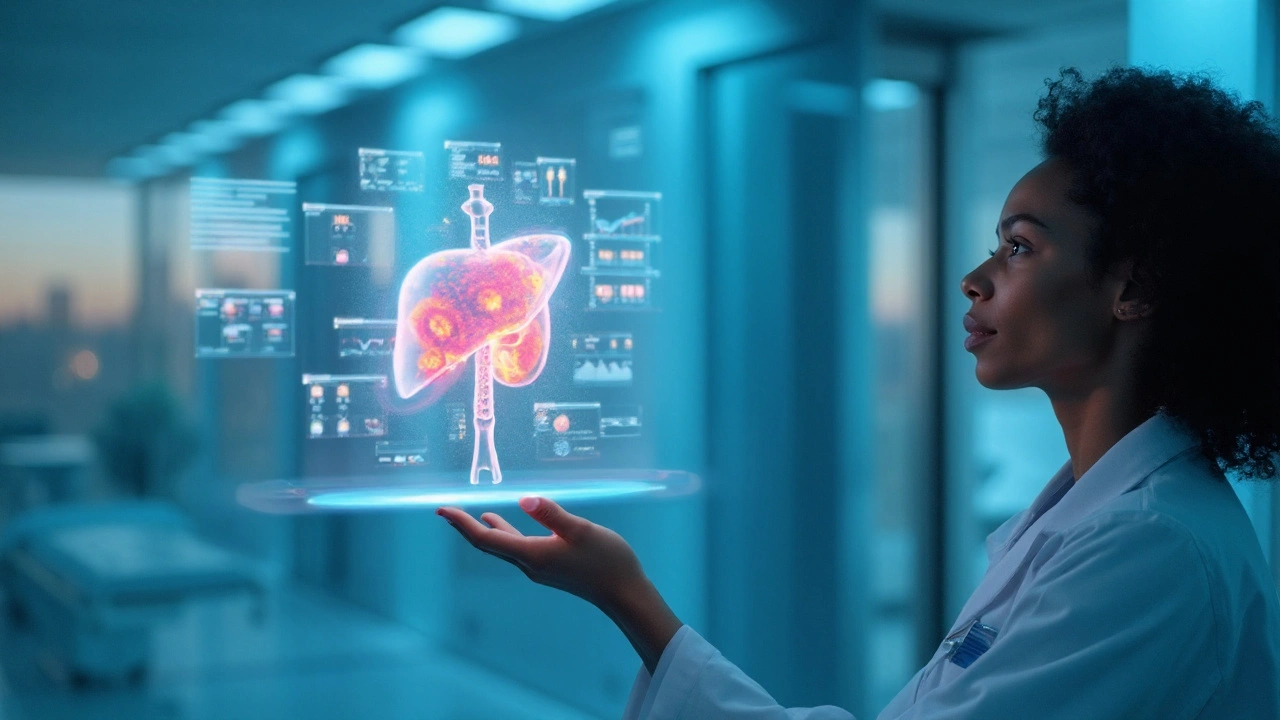
Liver cancer is a collective term for malignant tumours that originate in the liver, most commonly hepatic carcinoma, known as hepatocellular carcinoma (HCC). In 2023, over 900,000 new cases were diagnosed worldwide, and the five‑year survival rate remains under 20% in many regions. liver cancer research is now at a tipping point, driven by genomics, artificial intelligence, and novel drug platforms.
Why the Landscape Is Shifting
Three forces are converging to reshape how clinicians approach HCC. First, high‑throughput sequencing has mapped the mutational landscape, revealing driver genes such as TP53, CTNNB1, and TERT. Second, computational imaging powered by Artificial intelligence (AI) can detect micro‑vascular invasion on standard CT scans with 92% accuracy, a leap from radiologists’ 68%. Third, minimally invasive sampling-so‑called Liquid biopsy-captures circulating tumour DNA (ctDNA) and allows real‑time monitoring of treatment response. Together they promise earlier diagnosis, smarter trial design, and therapies that adapt to each tumour’s biology.
Emerging Therapeutic Modalities
Traditional systemic therapy relied on sorafenib, a multikinase inhibitor approved in 2007. Today, the pipeline includes three distinct classes that often overlap in clinical practice.
- Immunotherapy harnesses the patient’s own immune system. Checkpoint inhibitors such as atezolizumab, combined with bevacizumab, have shown a median overall‑survival (OS) gain of 7months over sorafenib.
- Targeted therapy blocks specific oncogenic pathways. Agents like lenvatinib and the newer tropomyosin receptor kinase (TRK) inhibitor selitrectinib target VEGF, FGFR, and MAPK signals, delivering response rates of 25‑30% in biomarker‑selected cohorts.
- Gene‑editing approaches such as CRISPR‑Cas9 are entering early‑phase trials. By knocking out mutant β‑catenin in patient‑derived organoids, researchers have achieved tumour regression in animal models, setting the stage for first‑in‑human studies.
Comparison of Key Emerging Therapies
| Modality | Mechanism | FDA approval (year) | Median OS improvement* | Typical grade≥3 side‑effects |
|---|---|---|---|---|
| Immunotherapy (atezolizumab+bevacizumab) | PD‑L1 blockade + VEGF inhibition | 2020 | +7months | Hypertension, bleeding |
| Targeted therapy (lenvatinib) | Multi‑kinase inhibition (VEGFR, FGFR, PDGFR) | 2018 | +3months | Hand‑foot syndrome, hypertension |
| CRISPR‑based gene editing (pre‑clinical) | Somatic knockout of oncogenic drivers | N/A | Pre‑clinical only | Off‑target edits (theoretical) |
| AI‑guided radiotherapy (proton beam) | Precision dose shaping using deep‑learning contouring | 2022 (FDA clearance for software) | +4months (in selected cohort) | Fatigue, skin erythema |
*Based on pivotal phaseIII trials or best‑available cohort data.
From Bench to Bedside: Model Systems Driving Discovery
Accurate disease models are essential for translating genomic insights into drugs. Three platforms now dominate HCC research.
- Patient‑derived organoids are three‑dimensional cultures that preserve tumour heterogeneity. A 2024 multicenter study showed that organoid drug‑response profiles matched clinical outcomes in 78% of cases.
- Humanized mouse models engrafted with a patient’s immune system enable testing of checkpoint inhibitors in a near‑human context. Results have identified biomarkers such as LAG‑3 expression that predict response.
- Spatial transcriptomics maps gene expression across tumour micro‑environments, revealing immune‑cold niches that may benefit combined radiotherapy‑immunotherapy approaches.
These systems feed data into AI pipelines, creating a feedback loop where computational predictions are experimentally validated, then refined.
Artificial Intelligence: The New Diagnostic Ally
Radiologists now rely on deep‑learning algorithms trained on millions of annotated liver scans. The most advanced models can:
- Distinguish HCC from benign lesions with >90% sensitivity.
- Predict micro‑vascular invasion before surgery, guiding transplant eligibility.
- Automate volumetric liver‑function assessments, reducing pre‑operative planning time from 45minutes to under 5.
Beyond imaging, AI curates electronic health records to flag patients eligible for clinical trials. In the United Kingdom, a pilot program at the Royal Marsden Hospital increased trial enrollment for liver‑cancer studies by 32% within six months.

Liquid Biopsy: Monitoring Tumours Without a Needle
Traditional surveillance relies on imaging every three months. ctDNA assays now detect tumour‑specific mutations at allele frequencies as low as 0.1%.
Key advantages include:
- Early detection of recurrence-median lead time of 4months over imaging.
- Dynamic assessment of resistance mechanisms, allowing rapid switch to second‑line targeted agents.
- Non‑invasive eligibility screening for trials focused on specific genetic alterations (e.g., NTRK fusions).
Regulatory bodies in the EU and US have granted breakthrough designation to several ctDNA platforms for liver cancer monitoring, signaling broader clinical adoption within the next two years.
Combination Strategies: The Future of Standard Care
Monotherapy rarely cures HCC, but rational combos are proving synergistic. The most promising regimens blend:
- Immune checkpoint blockade to unleash T‑cells.
- Anti‑angiogenic agents (e.g., bevacizumab) to normalize tumour vasculature, improving drug delivery.
- Localized therapies such as stereotactic body radiotherapy (SBRT) or radio‑embolization, which raise immunogenic cell death.
Recent phaseIII data (2023) showed that adding SBRT to atezolizumab+bevacizumab extended median progression‑free survival from 6.8months to 9.4months, with manageable toxicity.
Challenges and Ethical Considerations
Despite the excitement, several hurdles remain:
- Access disparity - high‑cost genomic testing and AI platforms are concentrated in tertiary centres, leaving rural patients behind.
- Data privacy - federated learning models protect patient data but require robust governance frameworks.
- Regulatory uncertainty - gene‑editing therapies fall between existing drug and biologic pathways, slowing trial initiation.
Stakeholders are calling for public‑private partnerships that subsidise sequencing for low‑income regions, and for transparent AI audit trails to maintain trust.
What Comes Next? A Roadmap for Patients and Clinicians
Within the next five years, you can expect:
- Standardised ctDNA panels integrated into surveillance guidelines.
- AI‑driven risk scores that dictate timing of liver transplantation.
- First‑in‑human CRISPR‑based tumour‑editing trials, potentially offering curative intent for early‑stage HCC.
- Regulated reimbursement models that bundle genomic testing, AI software, and targeted drugs.
For patients, the message is clear: ask your care team about molecular profiling and whether you qualify for combination immunotherapy trials. For clinicians, staying current with AI tools and trial eligibility criteria will become part of routine practice.
Frequently Asked Questions
What is the most common type of liver cancer?
Hepatocellular carcinoma (HCC) accounts for roughly 75-85% of primary liver cancers worldwide, often arising in the setting of chronic hepatitis B, hepatitis C, or cirrhosis.
How does liquid biopsy differ from a traditional liver biopsy?
A liquid biopsy analyses circulating tumour DNA (ctDNA) from a blood sample, detecting genetic mutations without the need for an invasive needle insertion. It can be repeated frequently, offering real‑time insight into tumour evolution.
Are AI tools approved for clinical use in liver cancer imaging?
Yes. In 2022 the FDA cleared a deep‑learning software that automatically segments liver lesions on CT scans. Adoption is growing, but clinicians must still review AI‑generated outputs.
What side‑effects should I expect from immunotherapy for HCC?
Common immune‑related events include fatigue, rash, and mild hepatitis. Severe (grade3‑4) reactions-such as colitis or pneumonitis-occur in about 10% of patients and are managed with steroids.
Is CRISPR therapy available for liver cancer patients?
Not yet. CRISPR‑based interventions are still in early‑phase (PhaseI) trials, primarily testing safety in solid tumours. Expect clinical availability only after comprehensive safety data emerge, likely beyond 2028.
Bob Martin
September 22, 2025 AT 05:09AI detecting micro-vascular invasion at 92% accuracy? Cool. But let’s be real - if the radiologist’s eyes are still the final arbiters, then we’re just automating confirmation bias. The tech’s good, but the human who clicks ‘approve’? That’s the weak link.
Billy Gambino
September 22, 2025 AT 09:26What we’re witnessing isn’t just a medical revolution - it’s a metaphysical shift. The tumor is no longer a discrete pathology, but a dynamic, self-modifying information system. CRISPR doesn’t edit DNA - it edits narrative. We’re not curing cancer anymore. We’re rewriting the story of cellular identity. And that’s terrifying. And beautiful.
Karen Werling
September 23, 2025 AT 08:51Yessss! 🙌 I’ve been waiting for this for years. My cousin had HCC and they did the liquid biopsy - no needle, no pain, just a vial of blood. It caught recurrence 4 months before the CT. I cried. This is hope with data behind it 💙
STEVEN SHELLEY
September 24, 2025 AT 00:55AI is just a tool for Big Pharma to hide the truth - they already have a cure but they’re keeping it secret because chemo and transplants make more money. They’re using CRISPR to make you dependent on their software. The FDA is owned by Merck. Look up the 2018 whistleblower memo. You think this is science? It’s a money laundering scheme with lab coats.
Emil Tompkins
September 24, 2025 AT 22:52Immunotherapy + bevacizumab extended PFS to 9.4 months? Wow. That’s like saying you got a 20% raise on a $10/hour job - still starving. And they call this progress? We’re just rearranging deck chairs on the Titanic while the rich get gene edits and the rest of us get a pamphlet and a prayer
Kevin Stone
September 25, 2025 AT 15:05People are celebrating AI and liquid biopsies like they’re magic. But have you looked at the cost? A single ctDNA panel runs $3,000. Most patients can’t afford it. This isn’t medicine - it’s luxury healthcare dressed up as innovation. The gap between rich and poor just got a genomic upgrade.
Natalie Eippert
September 26, 2025 AT 11:37Our country leads in medical innovation. Other nations are still using 1990s protocols while we’re editing genes. Why are we letting foreigners benefit from our breakthroughs? We should patent everything and export only to allies. This isn’t charity - it’s national security.
Tyler Mofield
September 27, 2025 AT 07:49The integration of spatial transcriptomics with AI-driven tumor microenvironment mapping represents a paradigmatic shift in oncological diagnostics. The confluence of multi-omic data streams enables predictive modeling at unprecedented resolution, thereby facilitating precision therapeutic stratification. The clinical translation of such platforms necessitates rigorous validation against gold-standard histopathological endpoints.
Patrick Dwyer
September 28, 2025 AT 05:09Just want to say - this is exactly why I went into hepatology. The organoid studies from 2024? Mind-blowing. When your patient’s tumor grows in a dish and responds the same way as in their body? That’s science with soul. We’re not just treating cancer anymore - we’re listening to it.
Bart Capoen
September 29, 2025 AT 02:26so i read this whole thing and honestly i’m just confused - like, if ai can detect invasion better than radiologists, why are we still doing biopsies? and why is crisper still in phase 1? also, can i get this stuff if i live in a town with no hospital? just asking for a friend who’s dying
luna dream
September 29, 2025 AT 04:08They say CRISPR is the future. But what if the tumor is just a symptom? What if cancer is the body’s way of screaming for balance? What if we’re not curing disease - we’re silencing nature’s warning system? The machines are listening, but are we?
Christy Tomerlin
September 29, 2025 AT 15:28Why is everyone so excited about AI? My uncle got scanned and the AI missed his tumor. Then the radiologist found it. So the AI’s not magic. It’s just another thing to blame when things go wrong.
Susan Karabin
September 29, 2025 AT 20:17This is the kind of stuff that makes me believe in science again. Not because it’s perfect - but because it’s trying. We’re getting better. Slowly. Messily. But better. Keep going. We need this.
Lorena Cabal Lopez
September 30, 2025 AT 14:09Too much jargon. Too many acronyms. I read half of this and fell asleep. Can someone just tell me if I’m gonna die or not?
Stuart Palley
October 1, 2025 AT 00:09They’re calling this breakthrough? I’ve seen this movie before. Every 5 years they say ‘we’re close’ - then they ask for more funding. I’m tired of being the lab rat. Where’s the cure? Or are we just funding tech demos for billionaires?
Glenda Walsh
October 1, 2025 AT 14:37Wait - so AI is reading CT scans now? What if it makes a mistake? Who’s liable? The hospital? The coder? The algorithm? What if it says ‘no cancer’ and someone dies? Are we ready for that? Are we really?
Tanuja Santhanakrishnan
October 2, 2025 AT 07:18I’m from India, and I’ve seen patients wait months for a biopsy. Liquid biopsy? Game-changer. No need to fly to the city. No need to pay for a surgeon. Just a blood draw. This could save lives in places where hospitals are 200km away. Thank you for writing this - it gives me hope.
Raj Modi
October 2, 2025 AT 09:42It is imperative to acknowledge that the convergence of genomics, artificial intelligence, and minimally invasive diagnostic modalities constitutes a transformative milestone in the management of hepatocellular carcinoma. The integration of patient-derived organoids with deep-learning algorithms enables the establishment of a closed-loop feedback system wherein in vitro pharmacodynamic responses are correlated with clinical outcomes, thereby facilitating personalized therapeutic decision-making. Furthermore, the regulatory pathways for gene-editing therapies remain inadequately defined, necessitating the development of harmonized international frameworks to ensure equitable access, patient safety, and scientific integrity.
Billy Gambino
October 2, 2025 AT 11:18You’re all missing the point. We’re not curing cancer. We’re outsourcing death to machines. The tumor doesn’t care about your algorithm. It just wants to live. And now we’re teaching it how to hide - better. The real breakthrough? We’re finally admitting we don’t understand life. We just want to control it.
Patrick Dwyer
October 2, 2025 AT 16:13That’s the thing - we’re not trying to control it. We’re trying to understand it. Every mutation, every signal, every echo in the tumor’s silence - it’s all data. And data can be read. We’re not gods. We’re translators. And this? This is the first sentence of a very long story.